
Imaging Deep Tissue Using Thin-Film Imagers
By Sarah Lindley
Media InquiriesFrom basic science to clinical diagnostics, optical imaging is a powerful route to answer biomedical questions.
But while something like detecting fluorescent proteins under a microscope is relatively straightforward, designing the best tools to image tissues deep in the body can get complicated.
Fiber optic endoscopes, for example, use bundles of optical fibers to relay images of tissues from the endoscope’s tip to a backend photodetector, each fiber transmitting one pixel.
While adding more fibers means more pixels, it also makes endoscopes larger and therefore more invasive. Though we typically think of optical fibers as rather thin, the bundles are still bulky enough for an endoscope to cause tissue damage when inserted. Fibers are also packaged into predefined honeycomb patterns, limiting flexibility in how the pixels can be arranged.
A novel, ultra-compact endoscope developed by a team led by Maysam Chamanzar, the Dr. William D. and Nancy W. Strecker Career Development Professor of Electrical and Computer Engineering, seeks to overcome these constraints. In a study recently published in Biomedical Optics Express, Chamanzar’s team presented Microimager, a thin-film endoscope that uses waveguides to detect light from deep tissues.
Like fibers, waveguides relay pixelized images of tissues to output ports, where they are received by backend photodetectors. Using a biocompatible photonics platform and an advanced microfabrication process, the researchers created tiny waveguides by surrounding a core of Parylene C—a polymer with a high refractive index—with a less refractive material for cladding.
But unlike fiber bundles, the waveguides’ layout is fully customizable. They can be arranged densely for local high resolution imaging, or more sparsely for lower resolution imaging of larger regions. Via a small area of rigid silicon that is purposefully retained under the backend during microfabrication, the Microimager can also directly integrate with optoelectronic systems, light sources, and surgical tools, making it exceedingly versatile.
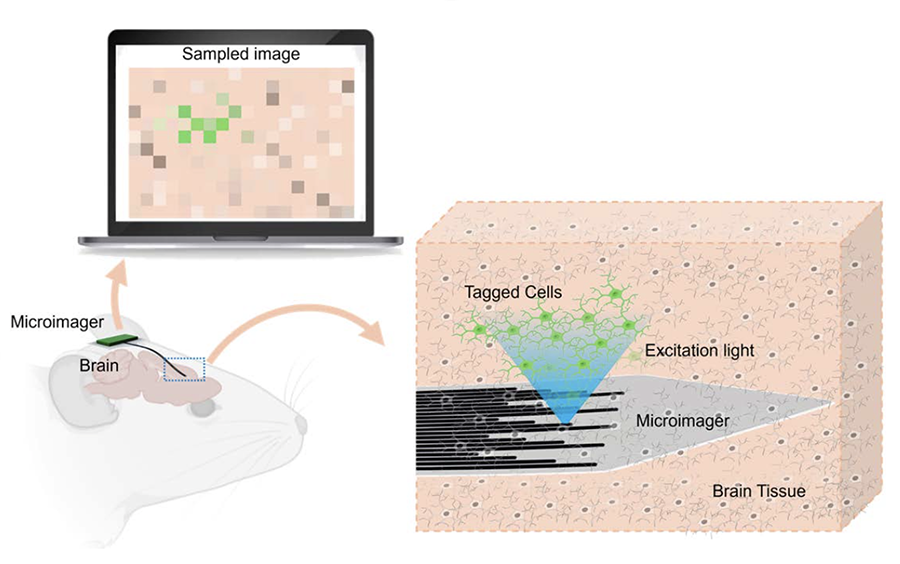
Chamanzar’s team envisioned a streamlined, waveguide-based design for the Microimager.
Source: Biomedical Optics Express
The Microimager is also much less invasive than other endoscopes. The shank, where the waveguides are housed, is only 400 microns wide and only seven microns thick. The waveguides are six to 10 millimeters long, resulting in an overall footprint more than 11 times smaller than the tiniest commercial light-measuring fiber.
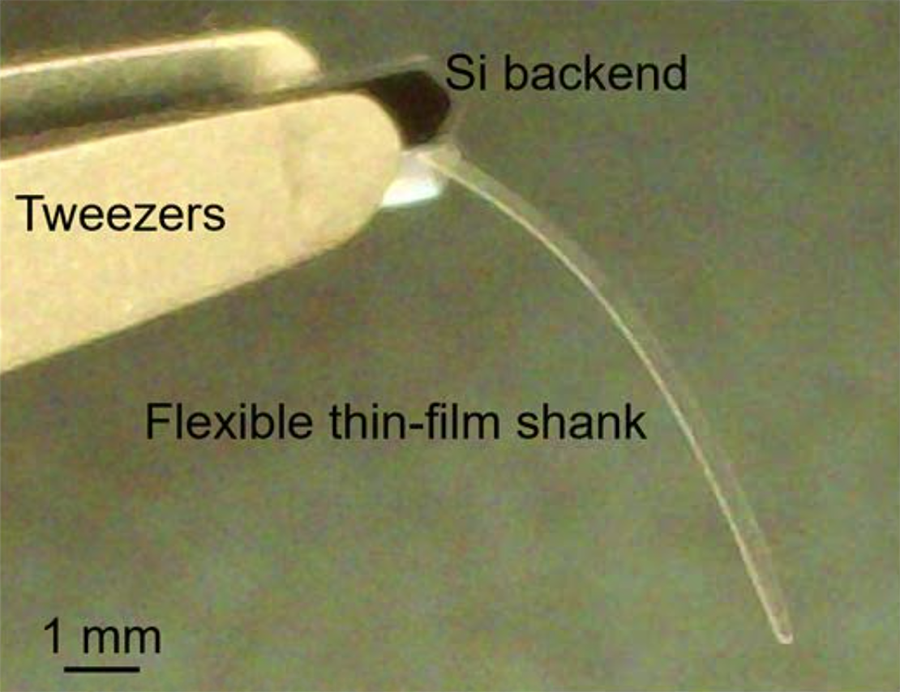 The Microimager is a uniquely small, minimally invasive endoscope.
The Microimager is a uniquely small, minimally invasive endoscope.
Source: Biomedical Optics Express
Chamanzar and his team, consisting of electrical and computer engineering graduate student Hassan Malekoshoaraie and neurobiology research scientist Vishal Jain, demonstrated the Microimager’s capabilities through a series of experiments. They placed microscopes with cameras at both the input and output ports, and positioned an optical fiber above the input to deliver blue light. That light can excite fluorescent microspheres and fluorescently-tagged proteins, producing green emission light that gets relayed through the thin-film imager and detected at the output microscope.
The team first conducted tests that confirmed that the Microimager’s inputs and outputs were accurately correlated, and that it could accurately relay patterns.
They then demonstrated that the Microimager is capable of 3D spatial discrimination. When the researchers embedded fluorescent microspheres in a gel at various heights above the waveguides, they could distinguish between them through differences in their output intensities.
Given these promising results, the researchers evaluated the Microimager’s performance in biological tissue. They harvested brain slices from mice that expressed green fluorescent protein (GFP) in their excitatory neurons, and kept these slices bathed in artificial cerebrospinal fluid to test and demonstrate the Microimager.
The GFP-expressing hippocampal regions emit light at different intensities, and these differences were reflected in the output. The Microimager could specifically discriminate between two regions with relatively high GFP expression, the stratum oriens and the stratum pyramidale, each with its own distinct output intensity peak.
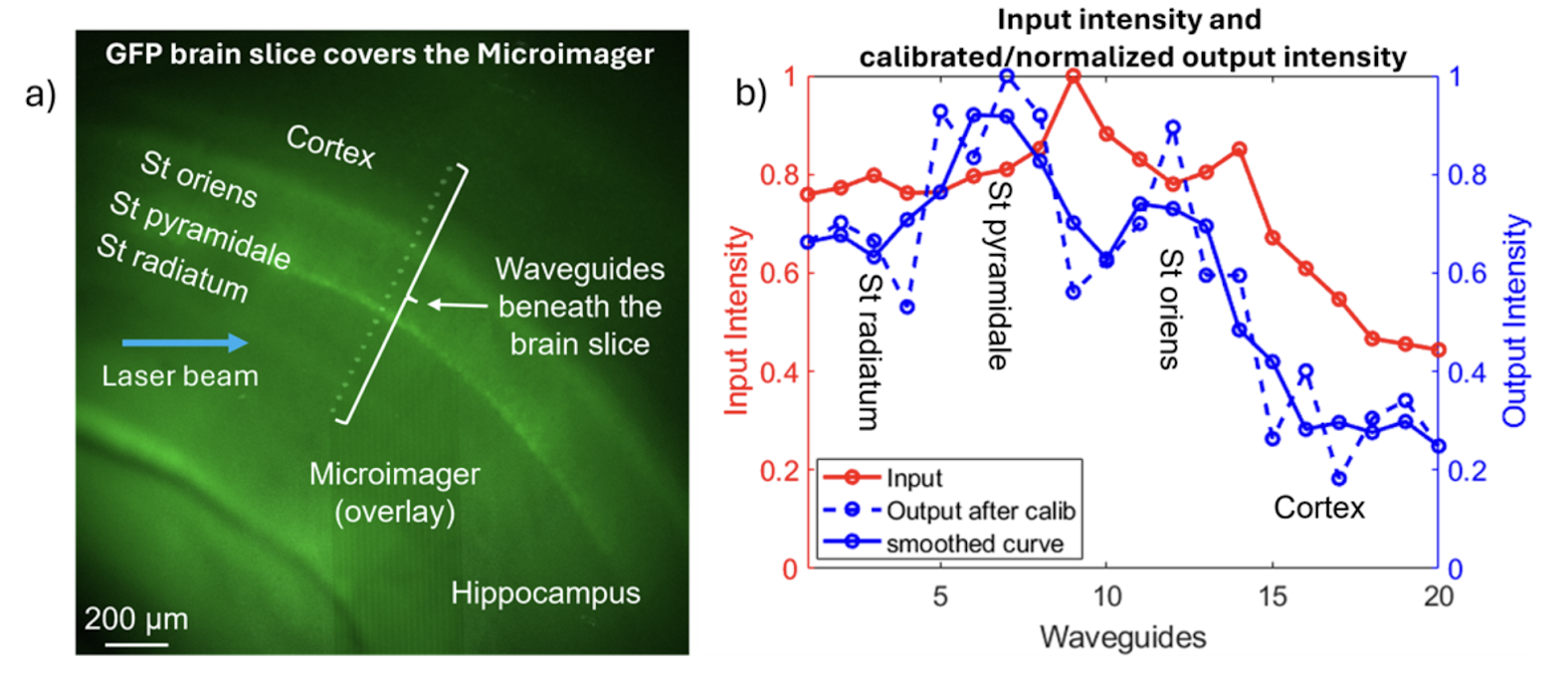 The Microimager can distinguish between the cerebral cortex and the hippocampal regions St. orients, St. pryamidale, and St. radiatum by producing distinct light output intensity
The Microimager can distinguish between the cerebral cortex and the hippocampal regions St. orients, St. pryamidale, and St. radiatum by producing distinct light output intensity
peaks for each.
Source: Biomedical Optics Express
Finally, the team demonstrated that the Microimager could detect temporal dynamics of brain function. By stimulating a particular layer of the cerebral cortex with an electrode, the researchers could induce calcium activity in specific neurons, causing them to emit GFP. When they stimulated layer V, the Microimager keyed them in on the response: a resulting increase in output light intensity in layers II and III.
Given the Microimager’s capabilities and flexibility for endoscopic imaging, Chamanzar’s team foresees many uses for it, from replacing traditional endoscopes used during surgeries to potentially implanting the Microimager into the brains of live, freely moving animals for research purposes.
As the team advances the microfabrication process even more, the tiny technology is poised to have an outsized effect on the field of optical imaging.